Arrotek Virtual Tour

Take a virtual tour of our medical device design and development facility. To find out more, please email us at [email protected].

Take a virtual tour of our medical device design and development facility. To find out more, please email us at [email protected].

You will probably work with a design partner to develop your idea for a new medical device. They will handle the design process, bringing the concept through to the initial prototype and then final prototype stage, ready for you to move to the next stage of your business plan. There are things that you can … Top Tips to Ensure a Successful Medical Device Design Process

Employees in Arrotek have taken part in an Eat Well, Feel Well workshop. It was presented by nutritionist Aoife Clancy. Aoife is the performance nutritionist with Sligo GAA’s Senior football and hurling teams. She also runs a nutrition clinic. The aim of the virtual workshop was to discuss a balanced approach to a healthy lifestyle, … Virtual Eat Well, Feel Well Workshop at Arrotek

Design verification is an important part of the medical device design and development process. It is an element that will be largely looked after by your medical device design partner. However, given it’s important to the overall process, it helps considerably if you have an overview of medical device design verification, why it’s important, and … Medical Device Design Verification – What You Need to Know

A product requirement specification outlines what needs to be achieved during the medical device design process. It is an important step in the process as it ensures design engineers fully understand what is required so they can deliver the best possible product. A good product requirement specification also makes the design process more efficient, and … 3 Essential Tips for Creating a Product Requirement Specification for a New Medical Device Idea

A crucial part of the medical device design and development process is to create a product requirement specification. The creation of this document takes place in the very earliest stages of the process. In fact, in our Six-Step Medical Device Design Process at Arrotek, creating the product requirement specification is one of the primary objectives … Establishing the Product Requirement Specification for Your New Medical Device Idea
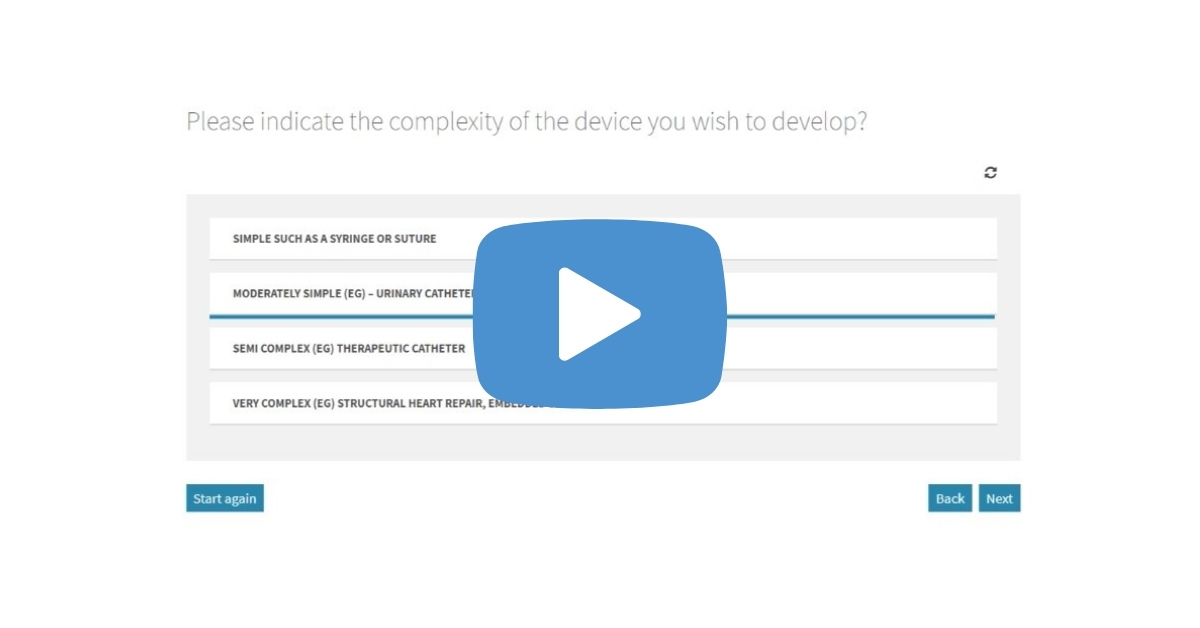
Our new video explains how to use the free Arrotek calculator tool to estimate the design and development costs that will be involved to turn your idea for a medical device into a new product. It only takes a few minutes to complete, and you don’t need to provide any commercially sensitive information. Watch the … Video: Estimating the Cost of Designing and Developing a new Medical Device

There are costs involved in designing and developing the idea you have for a medical device. Those costs can vary, however, depending on a range of factors. This includes the complexity of the device and the intended use. Understanding the costs of designing and developing your idea for a medical device is an important part … Step-By-Step Guide for Estimating the Cost of Developing Your New Medical Device Idea

The Arrotek team again this year got involved with the Nazareth House appeal. We received gift tags from Nazareth House containing the names of current residents. These are residents who have had minimal contact with loved ones throughout 2020 because of the impact of the pandemic. Nazareth House provides a range of different care services … Donating Christmas Gifts to Nazareth House

Charities always need support, but they need it even more so in 2020. That’s why we at Arrotek were determined to keep up our annual tradition of supporting local causes in a spirit of Christmas giving. The company has again this year donated to the Irish Kidney Association. It does vital work supporting patients affected … Supporting the Irish Kidney Association this Christmas