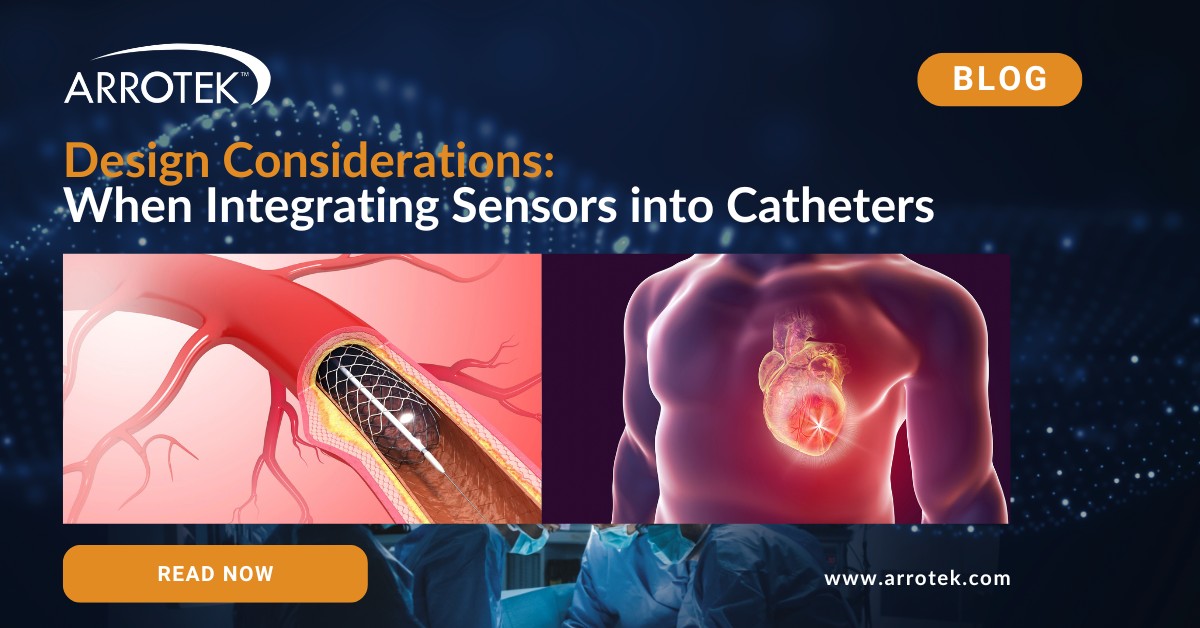
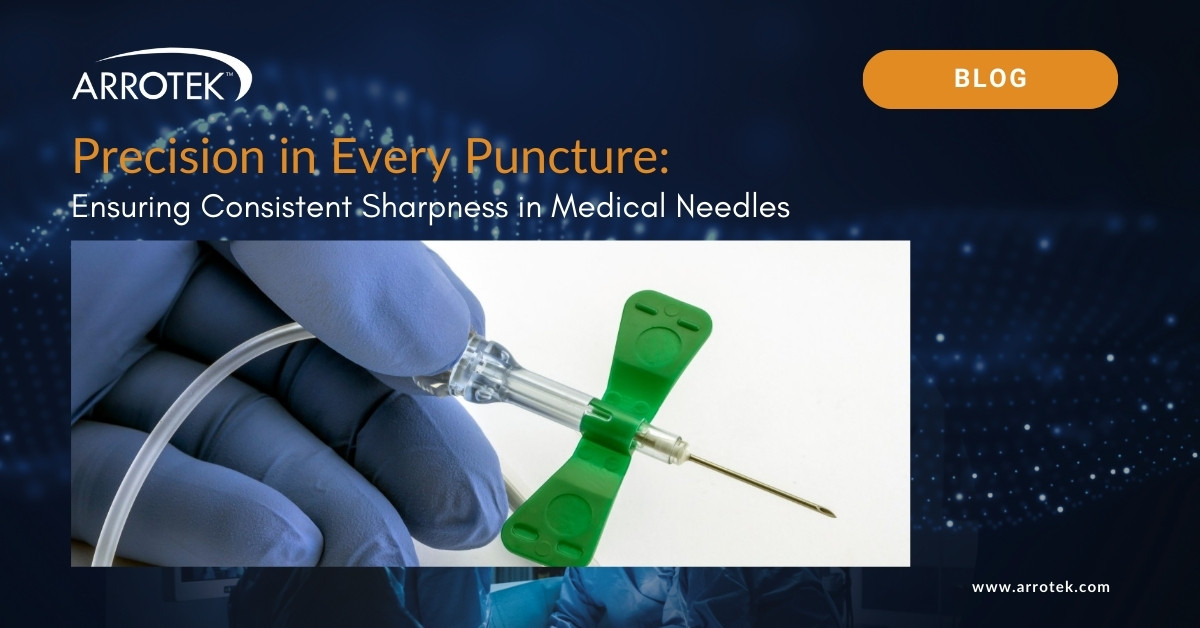
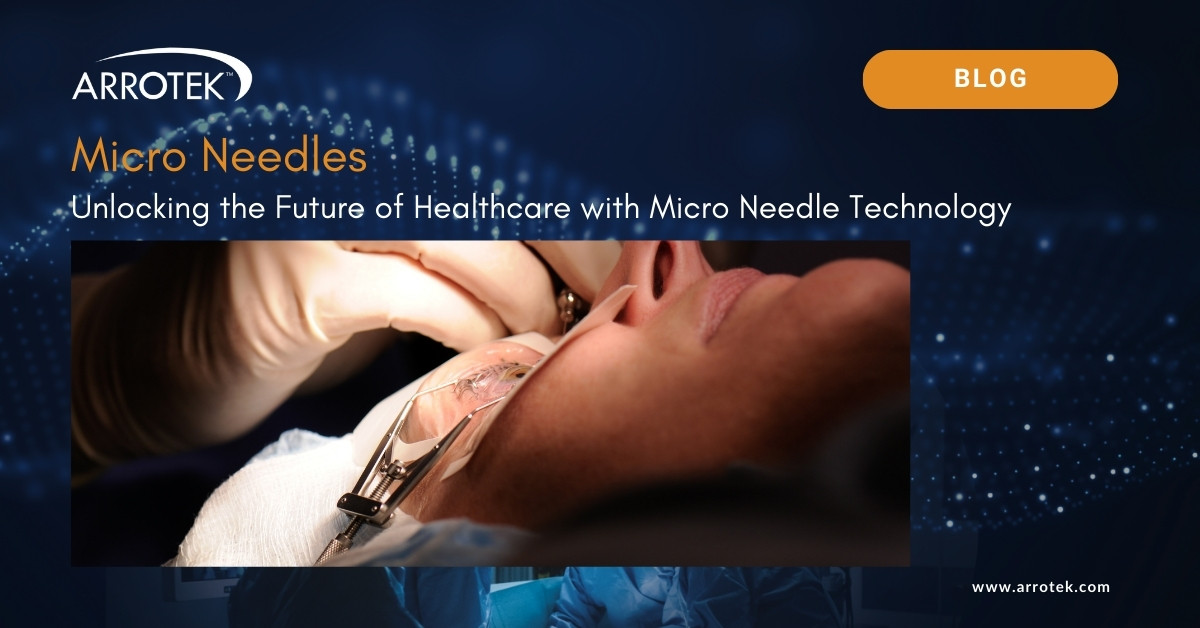
Brief Guide to Micropuncture Needles, Guidewires, and Catheters
The use of micropuncture needles, guidewires, and catheters has revolutionized many vascular access procedures. They provide an alternative to the

The use of micropuncture needles, guidewires, and catheters has revolutionized many vascular access procedures. They provide an alternative to the