There is a detailed and complex process involved in bringing an idea for a new medical device product from the early concept point through to a stage where it is being used by physicians in real-world clinical settings. The process includes all the typical elements you would find in any product design project. However, there are also regulatory considerations that need to be taken into account. Those considerations focus on minimising patient risk while ensuring the medical device is effective.
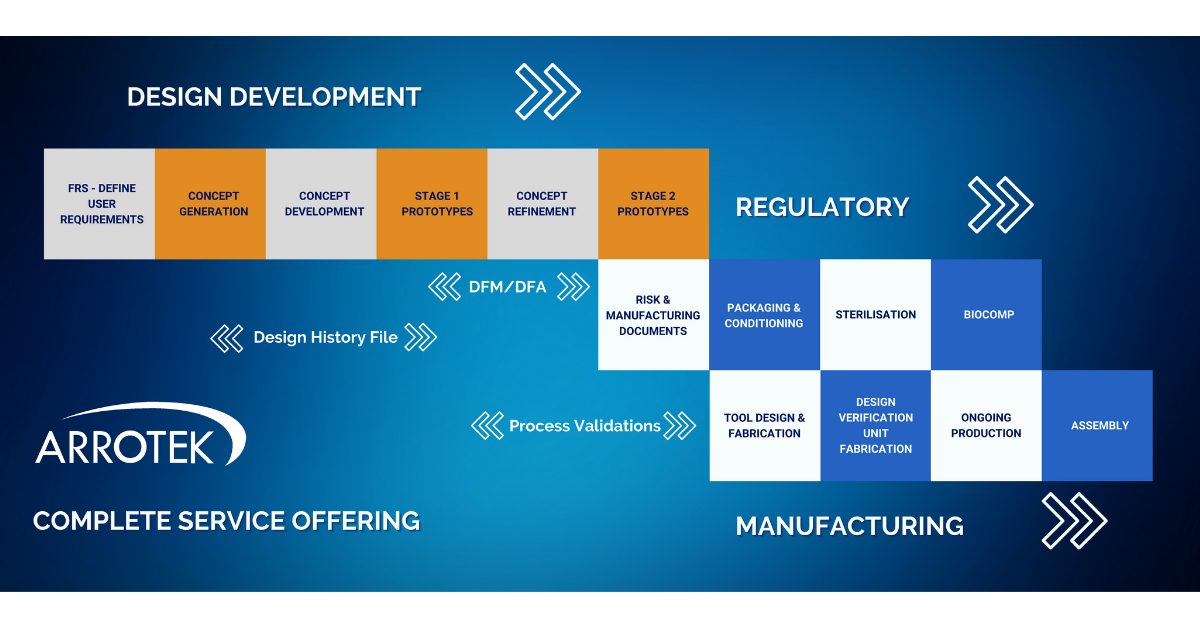
At Arrotek, we can offer a complete service offering. This means we can work with you to bring your idea for a new medical device through each of the three crucial phases:
- Design and development
- Regulatory
- Manufacturing
We have in-house expertise in all the above areas, as well as a state-of-the-art design and manufacturing facility. We also work with leading partners in the industry to fully optimise the process and ensure it is smooth and effective.
When we work with a customer on a medical device project, our relationship becomes a true partnership. It is a partnership where you retain full Intellectual Property rights and control over your product and idea, with complete confidentiality guaranteed.
Overview of Our Complete Service Offering
There are a number of crucial steps that take place as your idea for a new medical device progresses through the process of getting it to market. Those steps include consideration of regulatory documentation requirements and best practices in product design.
Importantly, we can get involved in your project at any stage. For example, we have experience becoming involved in catheter design projects that are through the initial Concept Development stage and are ready for the production of a Stage 1 Prototype. For us, every project is unique, so we fully customise our approach.
Design and Development Phase

The Design and Development phase of your project is covered by Arrotek’s well-established Six-Step Design Process. Those six steps include:
- Step 1: Defining – defining the functional requirements specification (FRS) and user requirements, documenting user needs, project planning, and initiating regulatory and design documentation.
- Step 2: Concept Generation – developing concepts that can deliver on the requirements and user needs defined in Step 1.
- Step 3: Concept Development – focus on one of the concepts from the previous stage, further developing that concept, creating a 3D CAD model, and reviewing materials and manufacturing methods.
- Step 4: Stage 1 Prototype – production of the first prototype ready for evaluation and testing.
- Step 5: Concept Refinement – refining the concept based on feedback and testing of the Stage 1 Prototype.
- Step 6: Stage 2 Prototype – production of a Stage 2 Prototype for further trials and testing.
A Design History File (DHF) will be continuously updated throughout the above six-step process. A DHF is a crucial document when making a submission for regulatory approval.
Validation and verification processes will be ongoing at various points in the above phase. Our design engineers will also begin looking at the manufacturing and assembly processes through Design for Manufacturing (DFM) and Design for Assembly (DFA) principles. Both DFM and DFA involve designing your product to ensure it is optimised for manufacturing and assembly.
Regulatory Phase

There is a lot of overlap through each of the phases. For example, our product designers start taking the manufacturing process into consideration early in the design phase, while maintaining regulatory documentation is always a crucial component.
That said, there is typically a clear shift from Design and Development to Regulatory once the Stage 2 Prototype stage has been reached.
Key stages in the Regulatory phase of the project include:
- Documentation – producing, updating, and refining required regulatory documentation, especially risk and manufacturing documentation.
- Packaging development – development of the packaging for your new medical device product with particular emphasis on atmospheric and environmental conditioning of the packaging, where we simulate real-world distribution conditions for testing purposes.
- Sterilisation – development and validation of the sterilisation method for your new medical device, including producing all the required documentation.
- Biocomp – biocompatibility testing according to guidelines and standards set out by regulators to ensure your new medical device product is safe for patients.
Our team will also work on the Manufacturing phase of your project alongside the Regulatory phase to ensure the overall process is as efficient as possible.
Manufacturing Phase

- Tool design and fabrication – design and development of the tooling required to produce your medical device product on a manufacturing production line.
- Design verification units – we’ll look after the process of manufacturing a small batch of your new medical device for design verification and testing purpose.
- Ongoing production – we have the facilities in-house to become your ongoing production partner with several cleanrooms on site encompassing class 7 and class 8.
- Assembly – to ensure the most efficient overall process, we also offer in-house assembly and packaging services to fully prepare your medical device product for distribution.
Let’s Discuss Your Project
Our complete service offering at Arrotek can not only move your project forward, but chart a clear path to regulatory approval, product launch, and ongoing production. We can offer as many or as few services as you need, and we’ll bring a wealth of experience to your project.
To speak to a member of our team today, please complete the form and we’ll get back to you.