Enhanced Rapid Prototyping Capabilities Available at Arrotek’s US Facility
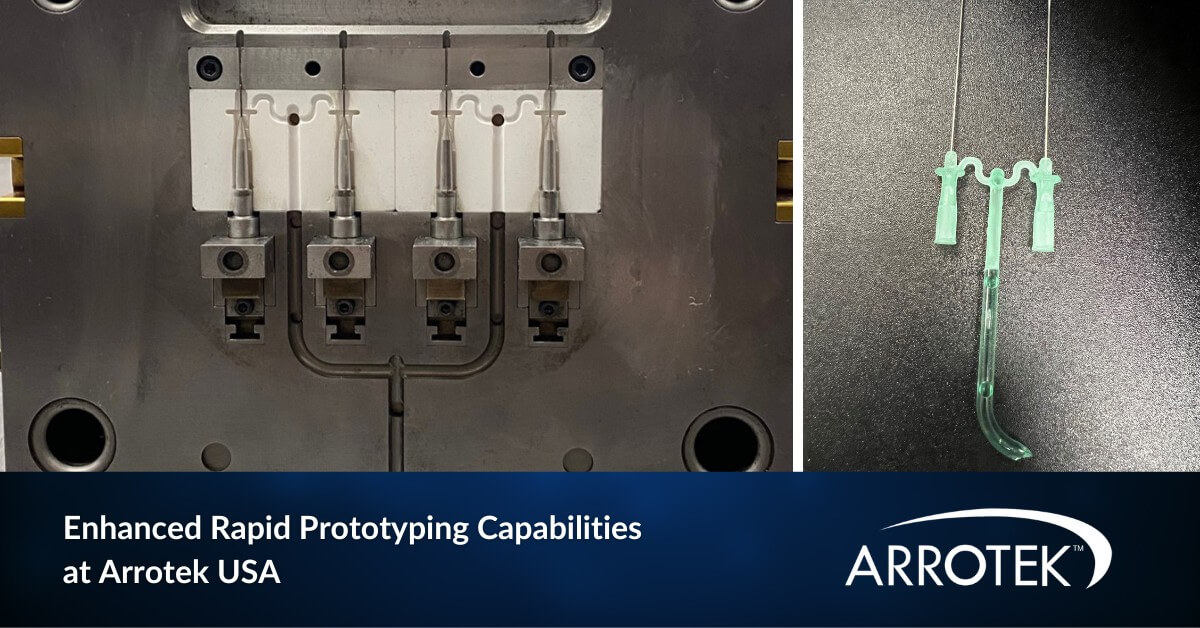
We have enhanced our medical device rapid prototyping capabilities at our US facility in Massachusetts. These increased capabilities will enable us to further improve the service we offer to customers, especially those in the design and development phase of their product’s lifecycle. At Arrotek USA, we can now provide customers with a fully functional prototype … Enhanced Rapid Prototyping Capabilities Available at Arrotek’s US Facility







