Whole Medical Device Design Expertise – Ensuring Your Project is a Success
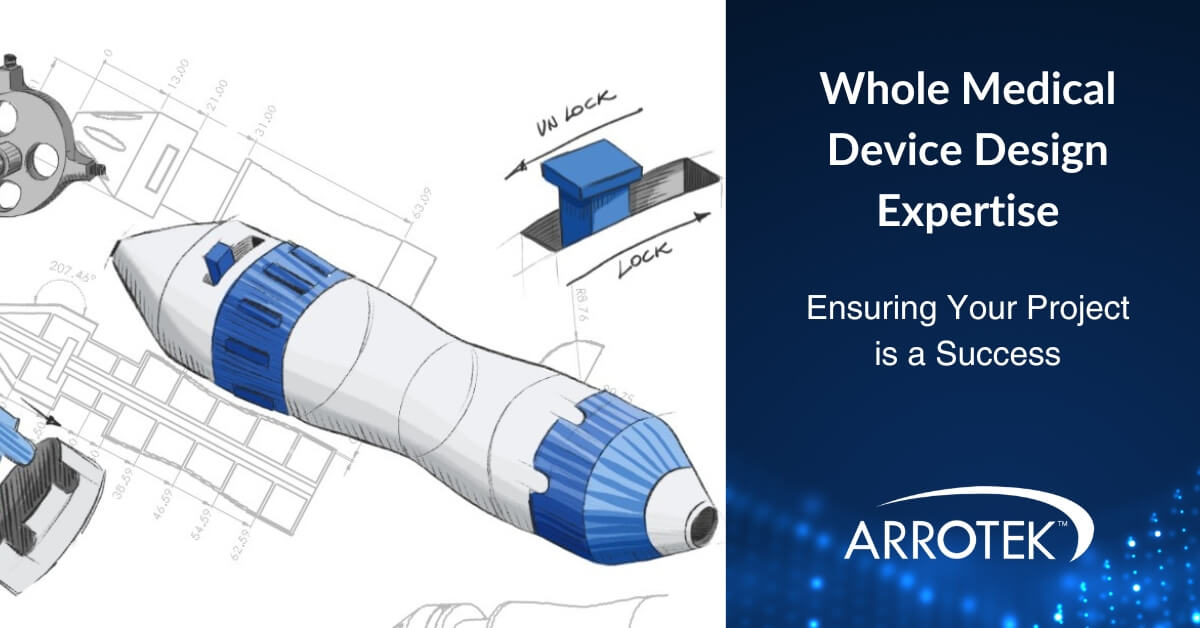
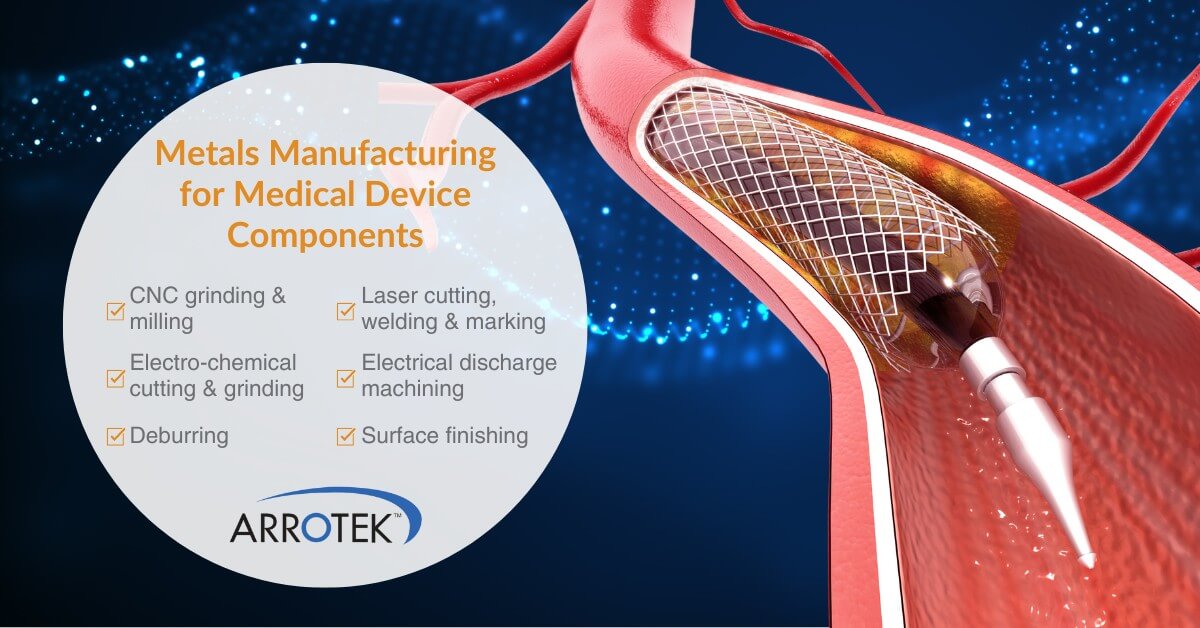
New and innovative medical devices are often complex with a range of different components and features. This particularly applies to advanced catheters, hand delivery systems, and other minimally invasive medical devices. Designing and developing those different components and features requires specific expertise and knowledge. Therefore, it is important to work with a medical device design … Whole Medical Device Design Expertise – Ensuring Your Project is a Success