
You have an idea for a new medical device. What do you do now? What are the next steps you should take? Each medical device development project is different, but there are common steps regarded as best practices in the industry. You might have already gone through some of the points below, but this will … Getting Started with Your Idea for a Medical Device

Update: Arrotek Technical Sales Manager Ronan Scott will be giving at talk at Virtual MedtecLIVE 2020 on the 6 Step Design Process for Medical Development. Get a ticket and find out more. This year’s MedtecLIVE event is going virtual, and Arrotek will be there as an exhibitor. MedtecLIVE is an annual event that brings together … Arrotek Exhibiting at Virtual MedtecLIVE

Dealing with a changed work environment as we do our part to help prevent the spread of COVID-19 isn’t just about homeworking and holding meetings on Zoom. Those things are important, of course, but what about those spur of the moment trips to the local cafe to grab lunch together, heading to the pub after … Virtual Bingo – Just One of the Ways Arrotek Has Adapted to the COVID-19 Lockdown

Last month, we put out a message on our website and on social media about our previous premises, a building we moved out of last year. It has 2,000 ft² of cleanroom space as well as other facilities suitable for medical device production. We offered this building free of charge to anyone who could use … COVID-19: Mask Production to Start at Previous Arrotek Facility in Sligo
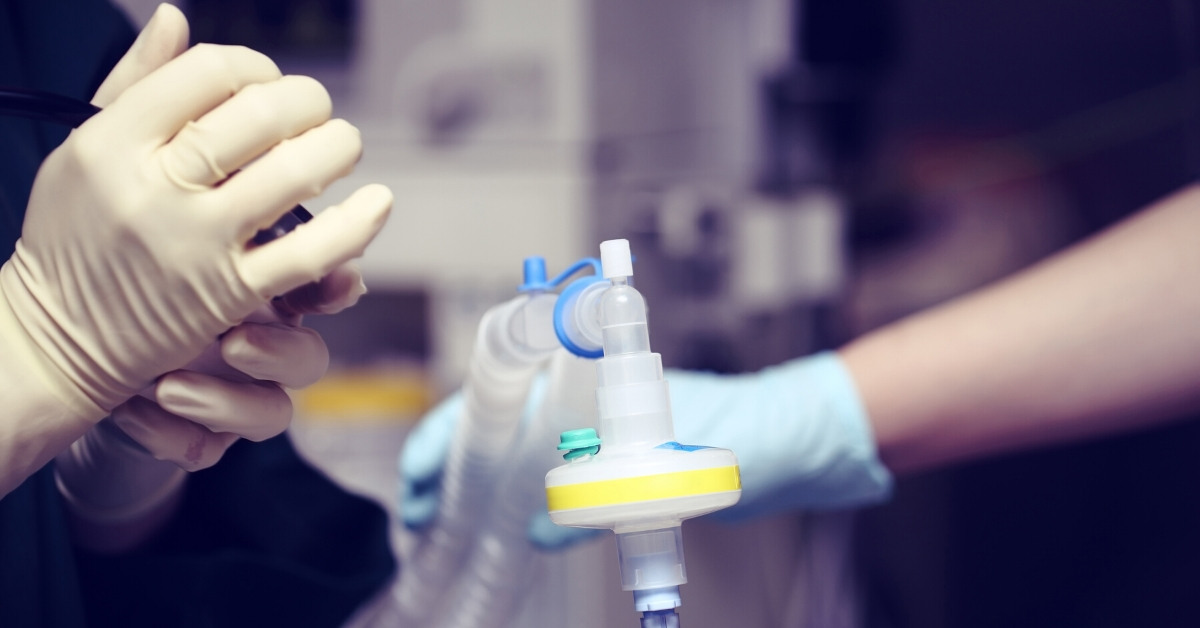
We’ve heard a lot in the news recently about PPE for healthcare staff – the equipment that protects them from COVID-19 infection. Arrotek is currently contributing to a project that aims to add an additional level of protection for those working on the hospital frontline. The Problem and the Solution Clinicians and other hospital workers … Arrotek Part of Collaborative Effort to Enhance Protection From COVID-19 for Frontline Healthcare Staff

Do you have an idea for a new medical device and want to know how much it will cost to get it from where you are now to the point that it is regulatory approved and ready for commercialisation? Do you also want to know the steps involved and the timescale that is likely to … How Much Will It Cost to Commercialise Your Idea for a New Medical Device?