An in-depth guide for medical device innovators and manufacturers
Transcatheter aortic valve implantation (TAVI) systems are now firmly established as a minimally invasive alternative to open-heart surgery for aortic stenosis. Also known as transcatheter aortic valve replacement (TAVR), these systems are continuing to evolve rapidly — with new materials, delivery systems, imaging and AI integration driving innovation.
In this article, we’ll review the state of TAVI system design in 2025, key design considerations, emerging trends, and what device manufacturers such as Arrotek must know when developing next-generation TAVI systems.
The market size, strong clinical uptake and expanding indications make TAVI systems an important growth area. Device innovation must keep pace with clinical and regulatory expectations (durability, outcomes, procedural efficiency, safety) as well as continue to enable minimally invasive approaches.
How TAVI systems are used
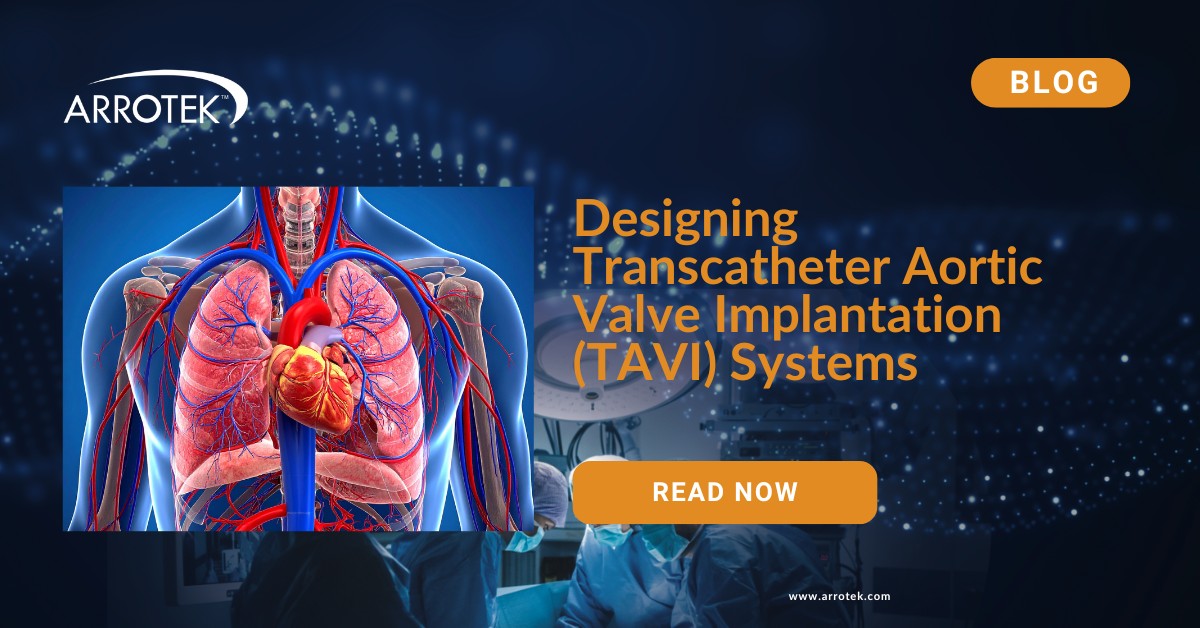
The aortic valve sits between the left ventricle and the aorta, when the valve becomes narrowed (aortic stenosis) the heart must work harder to pump blood into the body.
TAVI procedures introduce a replacement valve via catheter through a vascular access point (most commonly the femoral artery) and deploy the new valve in situ. The “trans-catheter” nature refers to the delivery via catheter rather than open chest surgery.
Alternate access routes (e.g., transapical, transaortic) are used in patients where femoral access is not feasible.
Key design considerations for TAVI systems
Designing a TAVI system involves many inter-dependent considerations. Below is a breakdown of major areas.
Valve type
Selecting the valve is foundational — it influences frame design, delivery system, deployment mechanics and long-term durability. Key types include:
- Balloon-expandable valves – a balloon is inflated to expand the valve frame.
- Self-expanding valves – often made of nitinol, these expand to their preset shape after deployment. (Nitinol’s shape-memory and elasticity make it ideal.)
- Mechanically expandable valves – newer designs where the operator uses mechanical control (rather than a balloon) to precisely expand the valve, enabling repositioning or partial deployment.
- Active-fixation valves – valves with additional fixation features (hooks, flaps, clasps, radial force) to improve stability and sealing, and reduce paravalvular leak.
Also positional variants:
- Supra-annular valves – sit above the native aortic annulus and maximise effective valve orifice area (improved hemodynamics).
- Intra-annular valves – fit within the annulus; may offer advantage in narrow anatomy or heavy calcification.
Delivery system and catheter design
Key delivery system design considerations include:
- Profile/size – Minimising catheter diameter reduces vascular trauma, broadens patient eligibility and may shorten hospital stay.
- Torque and control – Enhanced torque transmission ensures accurate navigation to the annulus and precise valve positioning.
- Markers and visibility – Radiopaque markers on the catheter and valve frame enhance imaging guidance and placement accuracy.
- Access path flexibility – Design must accommodate tortuous anatomy, calcification, varying access routes (femoral, transapical, alternative).
- Deployment control – Ability to reposition, recapture or partially deploy improves operator confidence and outcomes.
- Sealing and fixation – Integrated sealing skirts, frame geometry and fixation features help reduce paravalvular leak and need for pacemaker post-TAVI.
Materials and durability
Material selection is critical for valve longevity and biocompatibility:
- Bioprosthetic leaflets (bovine/pig) remain standard for most TAVI systems.
- Frame materials: nitinol, cobalt-chrome, stainless steel alloys. Frame design must balance radial strength, fatigue resistance, compactness and long-term durability.
- Delivery catheter materials need lubricity, kink resistance, trackability and low friction.
- Durability considerations: As more younger patients become eligible for TAVI, long-term durability (10-15+ years) becomes ever more important.
Imaging, planning & AI integration
In 2025, digital planning, AI/ML assistance and advanced imaging play an increasing role:
- CT-based morphology assessment of the aortic root and annulus helps refine sizing and reduce complications.
- AI/ML models are now being developed to predict post-TAVI pacemaker implantation risk.
- Robotic or assisted delivery systems are emerging to improve precision in valve orientation, alignment and positioning.
- For device designers, integrating digital-native features (data capture, imaging compatibility, AI-ready systems) can be a differentiator.
Clinical outcome optimisation
Design should aim to reduce key complications and improve procedural outcomes:
- Reduce paravalvular leak (PVL)
- Lower incidence of device-related conduction disturbances / need for pacemaker
- Enable shorter hospital stay / faster recovery (minimalist TAVI)
- Expand patient eligibility (narrow access, challenging anatomy, younger lower-risk patients)
- Improve long-term durability and haemodynamics
Emerging Trends & Future Directions (2025 and beyond)
In 2025 the TAVI field continues to evolve rapidly. Key trends include:
- Expansion of indications: TAVI is being applied in younger and lower-risk patients, and for other valve lesions (e.g., aortic regurgitation) beyond stenosis.
- Ultra-low profile delivery systems: Smaller sheath sizes increase vascular access options and allow wider patient eligibility.
- Durability and thrombosis resistance: With more patients expected to live many years post-TAVI, durability and leaflets’ long-term performance are under intense focus.
- AI and data analytics: Machine-learning based prediction of complications, imaging-guided sizing and real-time procedural analytics are becoming commonplace.
- Robotics and automation: Robotic-assisted catheter delivery and valve deployment systems are emerging, aiming for improved precision and lower operator variability.
- Patient-specific design and modularity: Customised valve/delivery system combinations, and modular platforms, allow adaptation to diverse anatomies.
- Minimalist procedural workflows: Same-day discharge, conscious sedation, single-artery access and shorter hospital stay are now mainstream in many centres.
- Imaging-device integration: Seamless connection between device, imaging system (CT/fluoroscopy/echo), and the planning software will increasingly differentiate systems.
What this means for Arrotek and device manufacturers
For a medical device innovator like Arrotek specialising in minimally invasive devices and advanced catheter technologies, the design of TAVI systems offers a high-value opportunity. Key considerations:
- Design for the future: Build platforms now that support ultra-low profiles, modular delivery systems, AI-ready data capture and multi-access routes.
- Focus on manufacturability: Valve frames, catheters, fixation systems must be designed with high-precision manufacturing, reliability and cost efficiency in mind.
- Materials and process selection: Manufacturing of nitinol frames, balloon-expandable options, coating technologies, and fulfilment of durability testing should be central.
- Clinical & regulatory readiness: With expanding indications and tighter long-term outcome expectations, the system must support regulatory longevity claims, and ease of use in real-world minimalist settings.
- Collaboration across domains: Device design must collaborate closely with imaging vendors, software/AI developers and clinical operators to deliver an integrated solution.
- Patient-centric and anatomically inclusive design: Designing for challenging anatomies (small femoral access, heavy calcification, younger patients) enhances addressable market and differentiates the device.
Conclusion
The design and manufacture of TAVI systems in 2025 is an exciting and high-growth area. With increasing clinical uptake, expanding indications and advancing technologies (AI, robotics, miniaturisation), the design bar is higher than ever. By aligning valve selection, delivery system design, material strategy, imaging integration and outcome-driven goals, device developers like Arrotek are well-positioned to succeed.
If you’re developing a TAVI system or delivery catheter, or exploring partnerships in advanced catheter technologies, our team at Arrotek have deep experience in minimally invasive medical device design and full-scale manufacturing. Get in touch to start a confidential discussion, email [email protected]
Related reads