And what we consistently see when teams move through this stage successfully.
Across our work with early-stage medtech teams, a familiar pattern tends to emerge.
A clear clinical need is identified. Early concepts take shape. Initial proof-of-concept models or early designs begin to form. At this point, progress often feels tangible and encouraging. Small teams move quickly, iteration is relatively inexpensive, and decisions can still be revisited without major consequence.
This phase is productive and necessary. It is also, in our experience, where expectations about how progress will continue are often set.
As projects move closer to prototype, the nature of the work changes. Decisions begin to carry downstream implications. Design choices start to influence regulatory pathways and manufacturing feasibility. The same approaches that worked well during early exploration begin to show their limits.
This transition is where many teams experience a loss of momentum.
The concept-to-prototype gap
The gap between a promising concept and a regulator-aware prototype is rarely defined by a single obstacle.
Instead, teams encounter a convergence of challenges that are difficult to resolve in isolation:
- multiple viable design directions, each with different trade-offs
- uncertainty around how regulatory expectations should influence early design decisions
- prototypes that demonstrate functionality but do not yet reduce future risk
- increasing pressure to demonstrate progress to external stakeholders
From our perspective, this stage is less about execution speed and more about decision clarity. Teams are still moving, but it becomes harder to determine whether effort is translating into readiness for the next phase.
Why teams stall here, even when progress is being made
Teams that reach this stage are rarely underperforming. More often, they are encountering a shift in complexity that requires a different way of working.
Decision density increases rapidly
As projects approach prototype, decisions that were previously independent begin to interact. Design geometry, material selection, usability, tolerances, and regulatory considerations start to overlap.
In practice, this often leads to decisions being revisited as new constraints surface. This is not a sign of poor decision-making. It reflects the increasing interdependence of choices at this stage.
Rework becomes more frequent and less visible
Iteration remains essential, but the cost of iteration changes. Early rework tends to be cheap and informative. Later rework often absorbs time and budget without proportionally reducing risk, especially when startups move forward without a clear structure for decisions and trade-offs.
We frequently see teams completing additional prototype cycles or refining specifications, while still feeling uncertain about whether those changes meaningfully advance the programme. For some, this is where structured, short-form formats like the Prototype Sprint can help bring clarity without overcommitting to a full development track.
This friction isn’t unique to startups. Larger medtech companies are facing the same challenge, and the ones pulling ahead are those investing earlier in structure, clarity, and integrated tooling to reduce delays downstream.
In Q4 of 2024, McKinsey highlighted that many of today’s top-performing medtechs are seeing success through “adoption of digital tools and solutions”, including iterative QA, simulation environments, and deeper design-data integration, often delivering a 20 percent reduction in development time, with a proportionate decrease in costs.
These insights reinforce a broader trend: teams that delay clarity usually pay for it later. Whether you’re a startup trying to get your first product to prototype or an enterprise scaling a pipeline, upstream uncertainty eventually shows up in the form of late rework, design conflicts, or slowed transfer to manufacturing.
Structuring this phase well, with decision-making frameworks, integrated expertise, and tools that surface friction early, is what separates velocity from drift.
Regulatory considerations surface earlier than expected
Many early-stage teams treat regulatory strategy as something to be addressed further downstream. From our experience working with medtech programmes, regulatory expectations often begin shaping feasibility and design decisions much earlier than anticipated.
When regulatory input enters the process late, design choices that initially appeared flexible can quickly become constraints. Teams are then required to revisit earlier assumptions, narrowing options at a point where change is more costly and harder to absorb.
In practice, this often shows up around fundamentals that feel straightforward early on. Intended use definitions, performance requirements, material choices, and manufacturability considerations are frequently explored in parallel, yet each carries regulatory implications that influence the others. Decisions made in isolation can unintentionally limit options later, particularly once design controls and approval pathways come into sharper focus.
Early regulatory alignment helps reduce downstream rework and avoids avoidable trade-offs later in development.
If you’d like a more detailed, device-specific example of how regulatory thinking, design decisions, and manufacturability intersect from the outset, our article “Catheter Design and Development – Key Considerations to Get Your Product to Market” explores this in more depth.
Fragmentation introduces hidden friction
If you’re working with multiple partners; maybe a design consultancy, a regulatory advisor, and a prototyping lab, you’re not alone. We see this setup all the time. On paper, things are moving: design files are progressing, documentation is in the works, and prototypes are getting built.
But behind the scenes, teams aren’t always speaking the same language or working toward the same decisions.
That kind of fragmentation can quietly stall momentum. One team might push for speed, while another’s focused on risk mitigation. Conversations happen in parallel, but the dots don’t always connect. And for founders or lean internal teams trying to stay on top of it all, it can feel like progress, just without the confidence that everything’s lining up.
We’ve seen this story play out enough to know that integration matters early. Without a joined-up process, trade-offs show up late, rework creeps in, and decisions have to be unpicked under pressure.
We touch on this more in our blog, where we unpack how alignment between functions drives better outcomes.
Progress becomes harder to measure
At this stage, teams are often under pressure to show momentum. Funding environments are tighter, and expectations around capital efficiency are higher than in previous cycles.
What constitutes “progress” becomes less obvious. Producing artefacts alone is no longer sufficient. Teams need confidence that effort is reducing risk and enabling the next step.
Recent industry analysis from EY’s Pulse of the MedTech Industry Report 2024 highlights the same dynamic at a macro level, noting that medtech companies are facing rising input costs, tighter reimbursement, and more constrained access to capital, which together create “a headwind on growth” and intensify scrutiny on how resources are deployed.
In this environment, EY argues that medtechs can no longer assume growth will follow automatically from innovation; instead, they must demonstrate that each investment in development directly supports sustainable revenue and margin, reinforcing the need for clear, risk‑based measures of progress rather than activity alone.
What teams that move through this stage do differently
Across programmes that progress cleanly from concept to prototype, a consistent set of behaviours tends to appear.
These teams introduce structure earlier than they initially feel is required. They narrow decision windows deliberately. Design, regulatory, and manufacturing considerations are aligned sooner, even when some uncertainty remains.
Rather than maximising optionality, the focus shifts toward reducing unknowns. Progress is defined by confidence gained, not just outputs completed.
This approach does not slow teams down. It changes where effort is applied.
From momentum to structured progress
This is the point at which many teams benefit from a more structured development approach.
Instead of addressing design, engineering, and regulatory considerations sequentially, they are considered together. Scope is defined upfront. Outcomes are tied to decision clarity rather than activity alone.
At Arrotek, the Design-to-Prototype Sprint was developed to support teams specifically at this transition point. It provides a fixed-scope, integrated pathway from concept toward a prototype, with regulatory thinking built in from the outset.
The aim is predictable progress. Teams know what decisions will be addressed, what outputs to expect, and how the work supports what comes next.
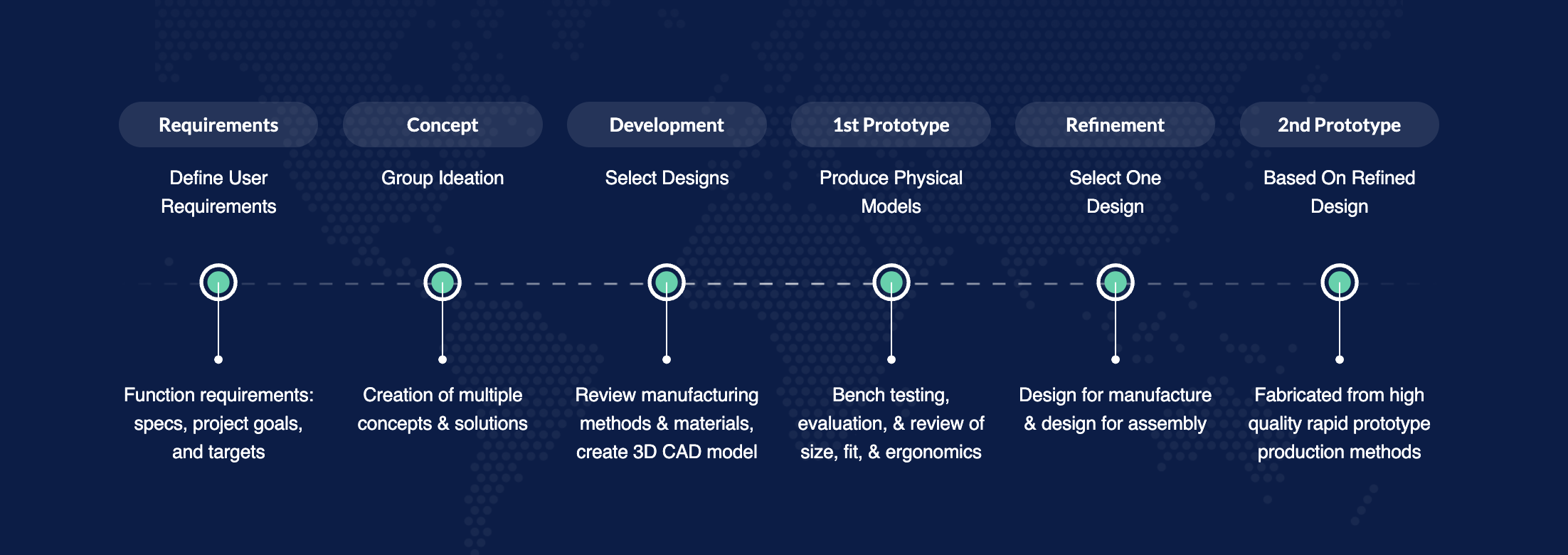
The Prototype Sprint Lifecycle
Why this stage matters downstream
How teams navigate the concept-to-prototype phase has lasting implications.
Early alignment at this stage supports:
- clearer regulatory planning
- more confident fundraising conversations
- smoother transition into scale or manufacturing
Unresolved uncertainty tends to resurface later, often when change becomes more costly.
For a deeper look at common early-stage challenges faced by startups, see how we support teams at different stages of development.
For teams already further along, the way this phase is handled often shapes how smoothly production and manufacturing scale-up can follow.
What this means in practice
Stalling between concept and prototype doesn’t mean a project is failing. More often, it reflects a natural increase in complexity, a signal that the development approach may need to evolve.
The risk at this stage is not slowing down. It is continuing without sufficient structure to ensure that progress translates into readiness.
For teams navigating this transition, the right framework early can determine whether momentum compounds or dissipates.
–
If you’re mapping your path toward prototype and weighing how best to reduce uncertainty at this stage, we’re always happy to explore what’s next, whether that’s helping shape your design inputs, advising on regulatory alignment, or finding the right first step to build momentum.





