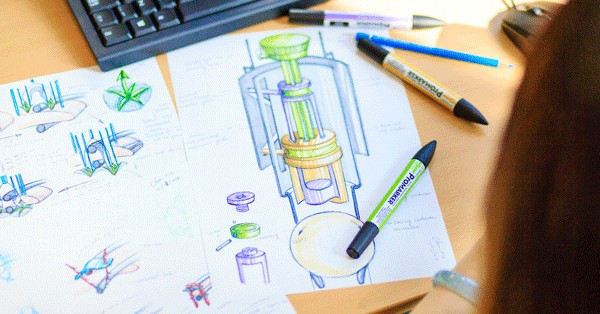
The best approach to developing a new medical device product is to use an iterative design process. Iterative design is a methodology used to create products in all industries, from physical products to software. What is iterative design, however, and what are the benefits?
What is Iterative Design?
Iterative design involves the development of a new medical device product using a cyclical process. This involves bringing the design to a certain point and then analysing, evaluating, testing, and getting feedback on that design.
This is all fed back to the design team to inform and shape the next stages of the process before repeating the cycle again.
Those stages include the basic concept stage, 3D modelling, and the creation of prototypes.
Why Use Iterative Design When Developing a New Medical Device Product?
An iterative design process ensures the product you want to develop goes through constant refinement and improvement as it is being designed. It involves multiple members of the design team working on your product.
At Arrotek, for example, we have people who specialise in various fields who contribute at crucial stages of our iterative design process.
An iterative design approach also involves you as the client.
In addition, it’s important the iterative design process starts at the very beginning of the development of a new medical device product. This is because changes are easiest and the least expensive to make when you identify and implement them at the beginning of a project.
The further into the development you go, the more expensive alterations become, even if the change is minor.
Benefits of Using Iterative Design
If you have an idea for a new medical device product, an iterative design approach to its development will deliver a range of benefits. This includes:
- Highlights and helps to resolve misunderstandings, expectation issues, and requirement inconsistencies as early in the process as possible
- Helps to ensure the product is fit for purpose and meets its functionality, usability, and reliability objectives
- Speeds up the design process, particularly of complex medical device products
- Keeps the design team focused on critical issues, helping them avoid distractions and diversions
- Improves the safety of the product
- Identifies previously unpredicted user behaviours which can lead to design changes. These user behaviours are almost impossible to spot without the 3D models and physical prototypes that are part of the iterative design process.
- Essential in the optimisation of DFM (Design for Manufacturing) which, in turn, ensures your product can be efficiently and cost-effectively manufactured at scale
- Identifies a range of usability and practical issues that are hard to identify early in the product design process without using an iterative approach.
- Ensures you are fully aware and up to date with the progress of the design and how your medical device idea is starting to shape up in reality
- Reduces the amount of reworking required, particularly in relation to clients not being happy with the design. As mentioned above, you as the client should be involved in the iterative design process from the very beginning, giving feedback and signing off on the design as it progresses through the various stages. By using this approach, major reworking of the product is avoided, reducing the overall design time.
- Helps ensure the product design process adheres to regulations by, for example, improving design control documentation
Get More Information
The specifics of an iterative design process depend on a range of factors including how you want to work, who is involved in the approval process, and the nature of the medical device product you want to develop.
To find out more about how those specifics relate to you, please contact us at Arrotek today.