2025 was a year defined by momentum across medical device design, development, and manufacturing. As the medtech sector continued to navigate regulatory complexity, rapid innovation cycles, and growing demand for minimally invasive technologies, Arrotek Medical’s work throughout the year reflected the broader direction of the industry — practical innovation grounded in engineering excellence.
This review captures the key themes, insights, and milestones that shaped the year.
Designing Devices with the Full Lifecycle in Mind
One of the clearest trends in 2025 was a shift toward end-to-end thinking in medical device development. Successful programmes increasingly depended on early alignment between design, regulatory strategy, and manufacturability.
Throughout the year, emphasis was placed on:
- Structured design processes that reduce risk and support regulatory compliance
- Prototyping strategies that balance speed with validation
- Designing from the outset for scale, reliability, and clinical usability
This lifecycle-driven approach became especially important as regulatory expectations continued to evolve across global markets.
Continued Focus on Catheter-Based & Cardiovascular Technologies
Minimally invasive and catheter-based devices remained at the forefront of innovation in 2025, particularly within cardiovascular and structural heart applications.
Key areas of focus included:
- Advanced catheter design, including material selection, performance optimisation, and testing
- Complex delivery systems for cardiovascular interventions such as TAVI
- The growing integration of sensors and smart features into catheter platforms
These developments reflected sustained market demand for technologies that improve patient outcomes while reducing procedural complexity.
Navigating Funding, Regulation & Market Readiness
Beyond engineering, 2025 highlighted how critical non-technical decisions are to medtech success.
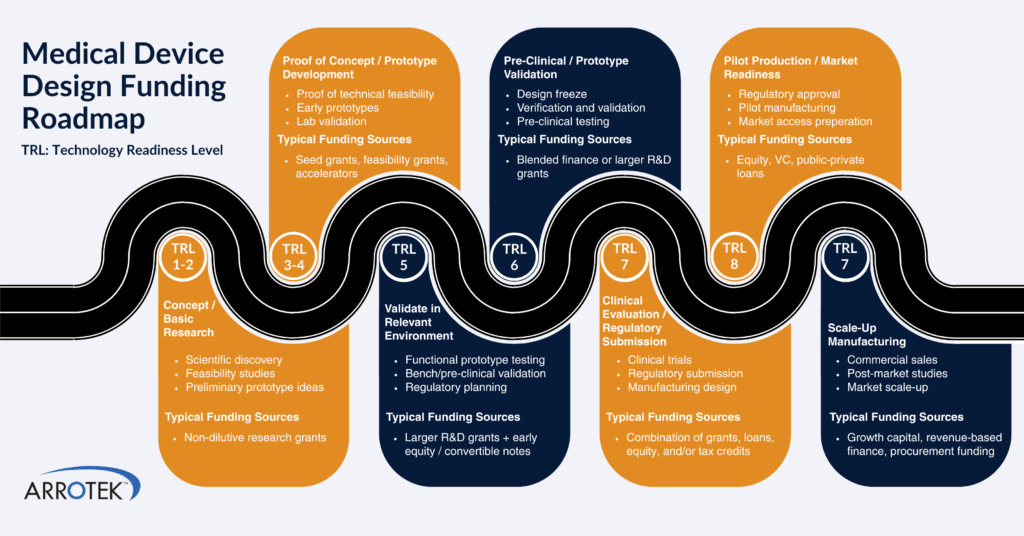
Across the year, attention centred on:
- Funding pathways for medical device companies operating in Europe
- The impact of early design choices on EU MDR compliance and time to market
- Aligning development strategy with long-term manufacturing and commercial goals
Together, these factors reinforced the importance of early planning and cross-functional decision-making.
Industry Engagement & Knowledge Exchange
Active participation in international medtech events and technical workshops played an important role in 2025. Engagements at major industry conferences and specialist catheter workshops created opportunities to:
- Share practical engineering experience
- Explore emerging trends in minimally invasive technologies
- Strengthen relationships across the global medtech ecosystem
These interactions helped keep innovation grounded in real-world clinical and manufacturing needs.
Recognition & Milestones in 2025
2025 was also a year of significant recognition and achievement for Arrotek Medical.
- Arrotek was shortlisted for multiple Life Science Industry Awards, including Life Science Company of the Year (Medium) and Best Engineering Project/Facility, reflecting strong performance in engineering and innovation.
- The company won Best Engineering Facility at the Life Science Industry Awards 2025, recognising investment in capability, quality, and technical excellence.
- Arrotek received a prestigious Supplier Award from Terumo, highlighting trusted partnerships and consistent delivery in highly regulated environments.
- Team milestones and long-service stories throughout the year underscored the role of experience, collaboration, and culture in sustaining innovation.
These achievements mirror wider industry priorities: quality, reliability, and long-term partnership.

Looking Ahead
As medical device development continues to evolve, the lessons from 2025 are clear: success remains rooted in strong engineering fundamentals, early strategic alignment, and collaboration across disciplines.
With growing demand for minimally invasive technologies and increasing regulatory complexity, the year ahead promises both challenges and opportunity.
Let’s Talk
If you’re planning a new medical device, refining an existing design, or preparing for manufacture in a regulated environment, now is the time to start the conversation.
Get in touch with the Arrotek Medical team to discuss how your next project can move forward with confidence, email [email protected]





