The introduction of the EU Medical Device Regulation (MDR) marked a major shift from the previous framework, the Medical Device Directive (MDD). Adapting to MDR has been – and remains – a substantial challenge for the industry. The implementation of the new regulations has faced persistent issues almost from the outset, compounding the difficulty of transition.
It’s fair to say that implementing EU MDR has been anything but smooth sailing. The whole industry, plus all stakeholders, have been affected – medical device companies, the institutions of the EU, notified bodies, national governments, healthcare providers, and patients.
Each part of the industry is working to adapt, even though capacity issues persist, systems are still not ready, and we are now long past the much-changed initial deadlines for implementation.
So, where are we? What are the remaining challenges and key developments? What are the main points you need to know?
Areas of Concern
The first point to highlight is that the EU MDR is not going away, and its stringent requirements are unlikely to be softened in any meaningful way. That said, there are significant areas of concern.
In particular, the challenges of EU MDR, consistently highlighted by the industry, are now firmly on the radar of EU legislators. The biggest concerns are:
- The potential impact on patients if critical medical device products become unavailable.
- The stifling of medical device innovation within the EU because of regulatory complexities and delays.
- The impact on medical device companies that often operate within competitive markets and challenging trading conditions.
- Limited notified body capacity leading to bottlenecks in conformity assessments.
Potential Reforms
The European Commission has launched a targeted evaluation of both the MDR and the In Vitro Diagnostic Medical Devices Regulation (IVDR). This evaluation is expected to inform future legislative amendments aimed at ensuring the regulations meet current and emerging needs, in addition to facilitating innovation. We expect to see the findings from the evaluation sometime in Q4 2025.
While no formal proposals have been adopted yet, several areas are under review and could lead to future reforms. Potential reforms and areas the EU could look to improve include:
- Streamlining the process for assessing and approving drug-device combination products
- Improving the coordination of clinical studies
- Expanding the rollout of electronic instructions for use (eIFUs) for medical devices
- Ensuring there is regulatory consistency across all EU member states
- Introducing special rules and procedures for rare disease medical devices (known as orphan medical devices) and paediatric medical devices to address unmet medical needs
- Considering the introduction of fast-track and prioritisation pathways to enable accelerated market access for breakthrough medical devices, especially where there are unmet needs
- Supporting SME medical device companies where the cost of transitioning to EU MDR is disproportionately burdensome compared to large corporations.
EUDAMED
Greater clarity is expected in the coming months on the introduction of EUDAMED – the European Database on Medical Devices. It is still not fully operational, but the European Commission has confirmed it will be implemented on a phased roll-out basis. This will be based on the modules within the database:
- Actor registration
- UDI/device registration
- Notified bodies and certificates
- Clinical investigations and performance studies
- Vigilance and post-market surveillance
- Market surveillance
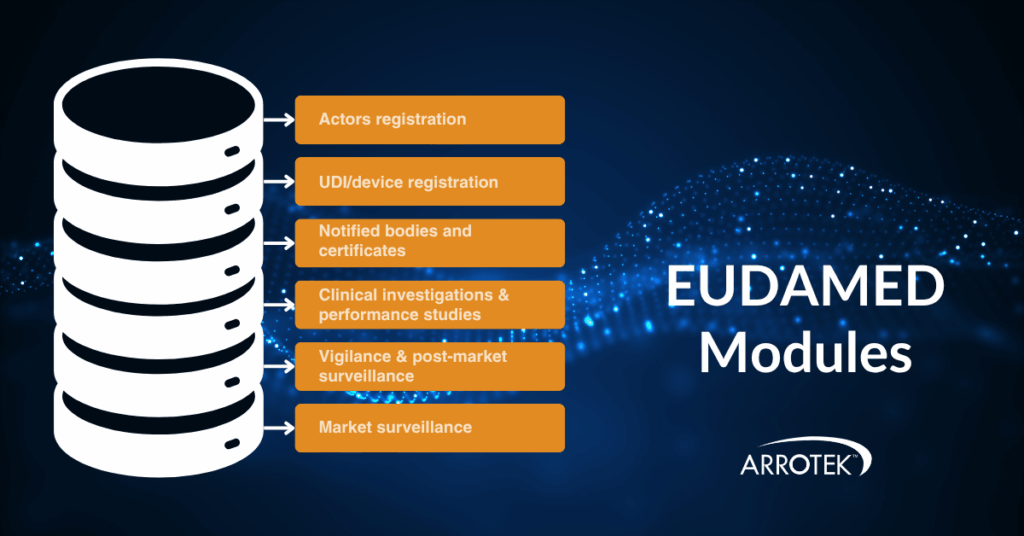
It is expected the first four modules will become mandatory in Q1 2026, possibly as early as 1 January 2026. The remaining two modules (vigilance and post-market surveillance, plus market surveillance) will become mandatory in a future phase of the roll-out.
Transition Deadlines
One of the requirements under EU MDR is that all existing devices on the EU market must be transitioned to the new regulations by undergoing a conformity assessment procedure.
However, because of capacity issues and other problems in the system, the originally published deadline for this transition has been pushed back. The exact deadline that now applies depends on the class of device:
- Class III and Class IIb implantable devices – the deadline for transition to EU MDR is 31 December 2027.
- Other Class II devices, as well as Class IIa and Class I devices – the deadline for transition to EU MDR is 31 December 2028.
Until these deadlines are reached, legacy devices with valid MDD certificates may continue to be marketed in the EU, provided they meet certain conditions outlined in Article 120 of MDR.

Conclusion: A Lot Has Happened, A Lot Hasn’t, and There’s More to Come
The changing parameters of EU MDR, the pushed back deadlines, and the delays have been challenging, but they have also demonstrated the resilience, adaptability, and flexibility of the medical device industry in the EU.
We used a phrase at the start of this blog about progress not being smooth sailing so far. We are still not in calm waters, to keep the metaphor going, so further resilience, adaptability, and flexibility will be required.
There are also positives for the industry, such as the expected initiatives to facilitate and enhance innovation within the confines of the regulations.
The key piece of advice for medical device product owners and manufacturers is not to stand still and not to wait until everything is settled before taking action. Being proactive about EU MDR is the best way forward.
Need Help Navigating EU MDR?
If your company has questions or concerns about EU MDR compliance, timelines, or how upcoming reforms might affect your products, our team at Arrotek is here to help.Contact us today to arrange a consultation.





