2025 is a year full of opportunity for innovation and growth, especially in the medical device industry. While businesses across all sectors face ongoing challenges such as tariffs, economic shifts, and supply chain disruptions, these headwinds are also driving fresh thinking and progress. For medical device companies, now is the ideal time to move forward with your design project and turn today’s challenges into tomorrow’s breakthroughs.
In this blog, we will outline the reasons for our positive outlook on medical device innovation in 2025. As a summary, regulatory momentum is shifting in favour of innovation, especially in the EU, with streamlined compliance pathways. You can also get a competitive advantage by moving forward in a growing market with active investment and M&A activity. Plus, you can take advantage of new technologies and partnership opportunities before your competitors.
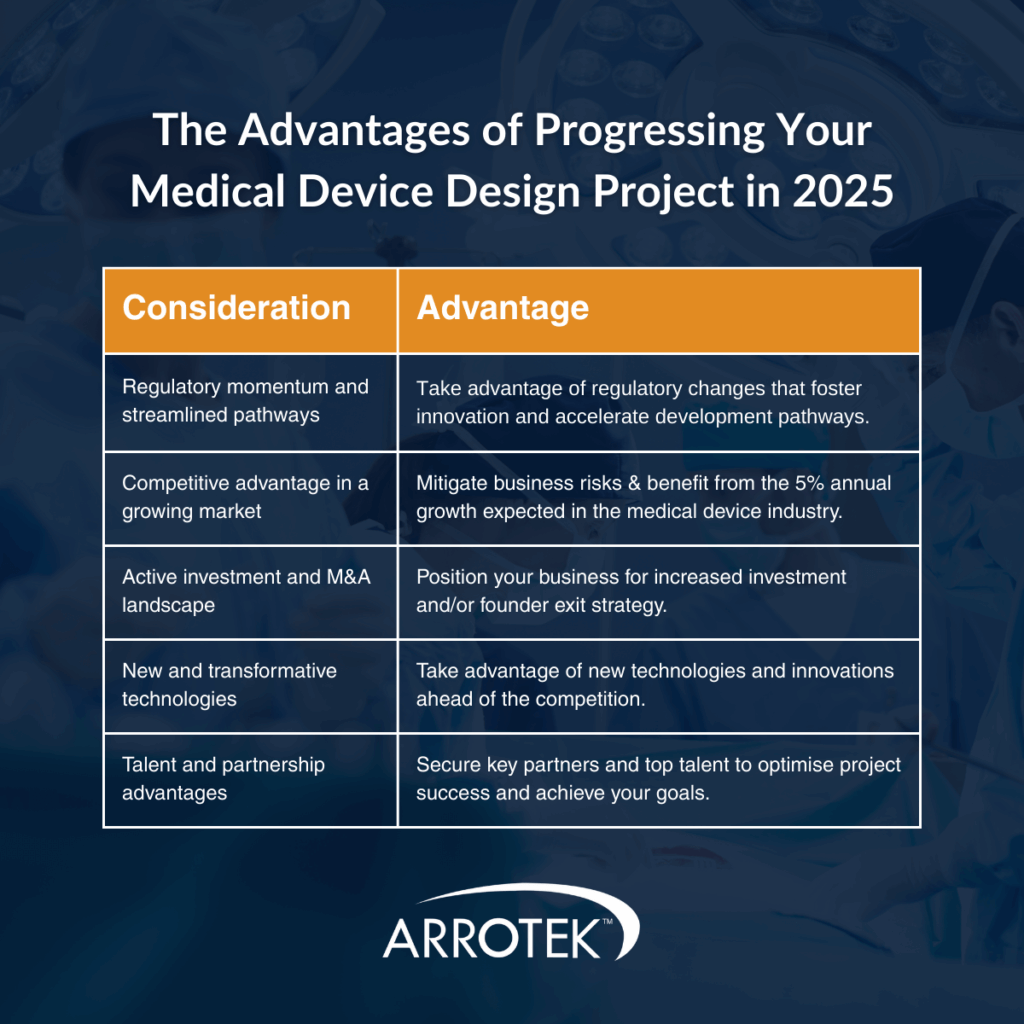
1. Regulatory Momentum and Streamlined Pathways
The EU is updating the regulatory environment that medical device companies must operate within, including EU MDR (Medical Device Regulations). The changes are in response to industry concerns, with some already implemented, such as the introduction of the Health Technology Assessment Regulation (HTAR).
As a result of recent consultations, including a targeted EU MDR evaluation, more amendments and refinements are expected. Key aims of the reforms include:
- Fostering innovation
- Reducing administrative burdens
- Enhancing support for small and medium-sized companies
- Accelerating development pathways for new and advanced medical device technologies
2. Competitive Advantage in a Growing Market
Tariffs, other economic barriers, and the complexity and volatility of the current geopolitical environment are disrupting parts of the medical device industry. There are also significant opportunities. Factors to consider in your decision-making include:
- Tariffs don’t impact medical device design and development projects, giving you time to make decisions on where your finished product will be manufactured. Partnering with a company like Arrotek ensures you have multiple options, as we have manufacturing facilities in the EU, the US, and Central America.
- While market conditions are unpredictable, what is not unpredictable is demand. People continue to get sick, develop chronic conditions, and require medical assessments and intervention.
- Some supply chains have suffered disruption, but the medical device industry has proven to be highly resilient and versatile. You can also take steps to significantly mitigate disruption by working with partners experienced in supply chain management.
- New innovations and novel devices are less impacted by market and economic fluctuations as they often escape tariffs and other trade barriers.
It is also important to point out that, despite geopolitical, trade, and economic concerns, the medical device industry is still expected to grow by 5 percent a year up to 2030.

This predicted growth is driven by a range of factors, many of which are outside the remit of politics and economics. This includes aging populations, especially in European countries, alongside a rise in the prevalence of chronic diseases.
Early movers can capture market share, build brand recognition, and establish partnerships before the market becomes more crowded.
3. Active Investment and M&A Landscape
While some investors took a “wait and see” approach in Q1 2025 as developments in the US unfolded, venture capital and private equity investments are now rising, fuelling R&D and innovation. Furthermore, declining interest rates and strong cash positions among leading MedTech companies are driving a more active environment for mergers and acquisitions.
4. New and Transformative Technologies
New and transformative technologies are creating significant opportunities for medical device innovation. Examples include:
- Developments in material science and engineering are driving innovation in minimally invasive medical devices.
- Sensors, fibre optics, the internet of medical things (IoMT), and other digital technologies.
- Artificial intelligence and machine learning algorithms.
- Combination products that have both medical device and pharmaceutical components.
Companies starting now can leverage these technologies ahead of competitors, establishing leadership positions in rapidly growing segments such as wearables and minimally invasive devices.
5. Talent and Partnership Advantages
The current surge in MedTech innovation attracts top talent and fosters new collaborations. Starting now allows your company to secure key partners and skilled personnel before demand intensifies further.
Summary: Progressing Your Medical Device Design Project in 2025
Conclusion: 2025 is the Year of Medical Device Design and Innovation
The industry is growing, the regulatory landscape is becoming more favourable to product innovation, and there is an active investment and M&A environment. These are just some of the reasons making 2025 the right time for taking the next steps in your medical device design and development project. As specialists in minimally invasive medical device design, we can help you take full advantage of the opportunities that currently exist. Get in touch with us at Arrotek today to learn more.





